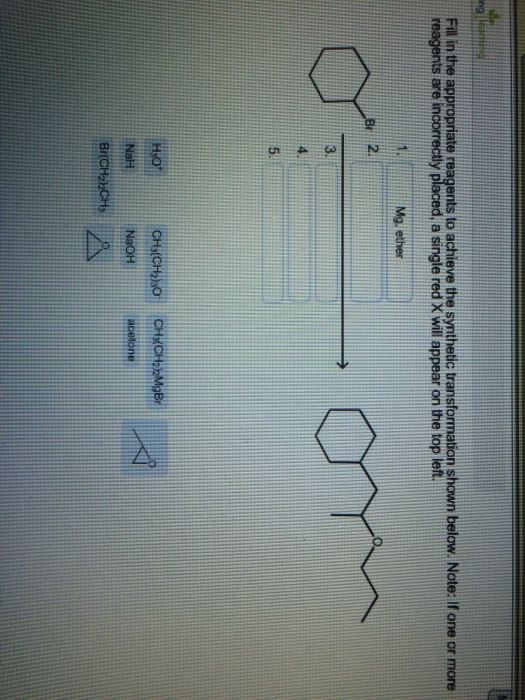
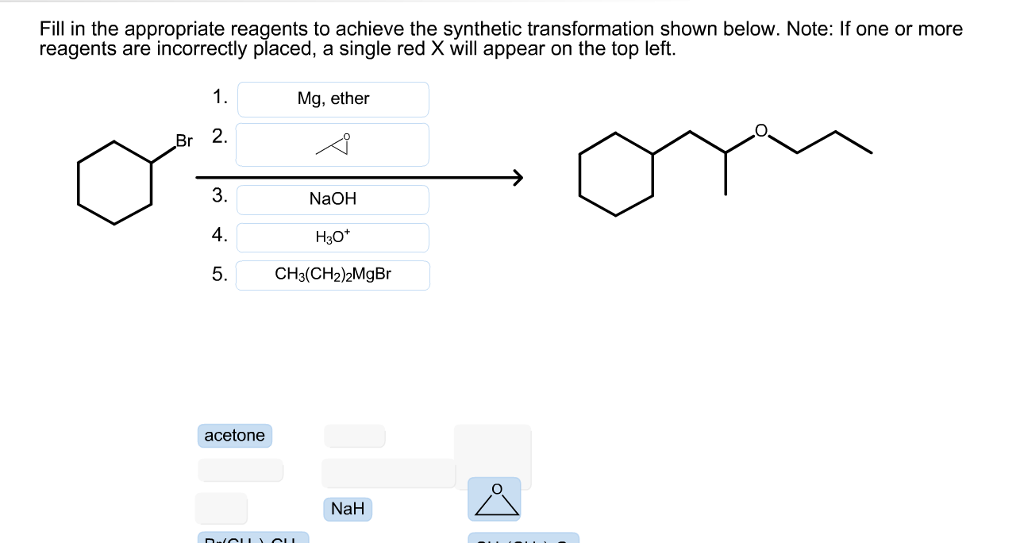
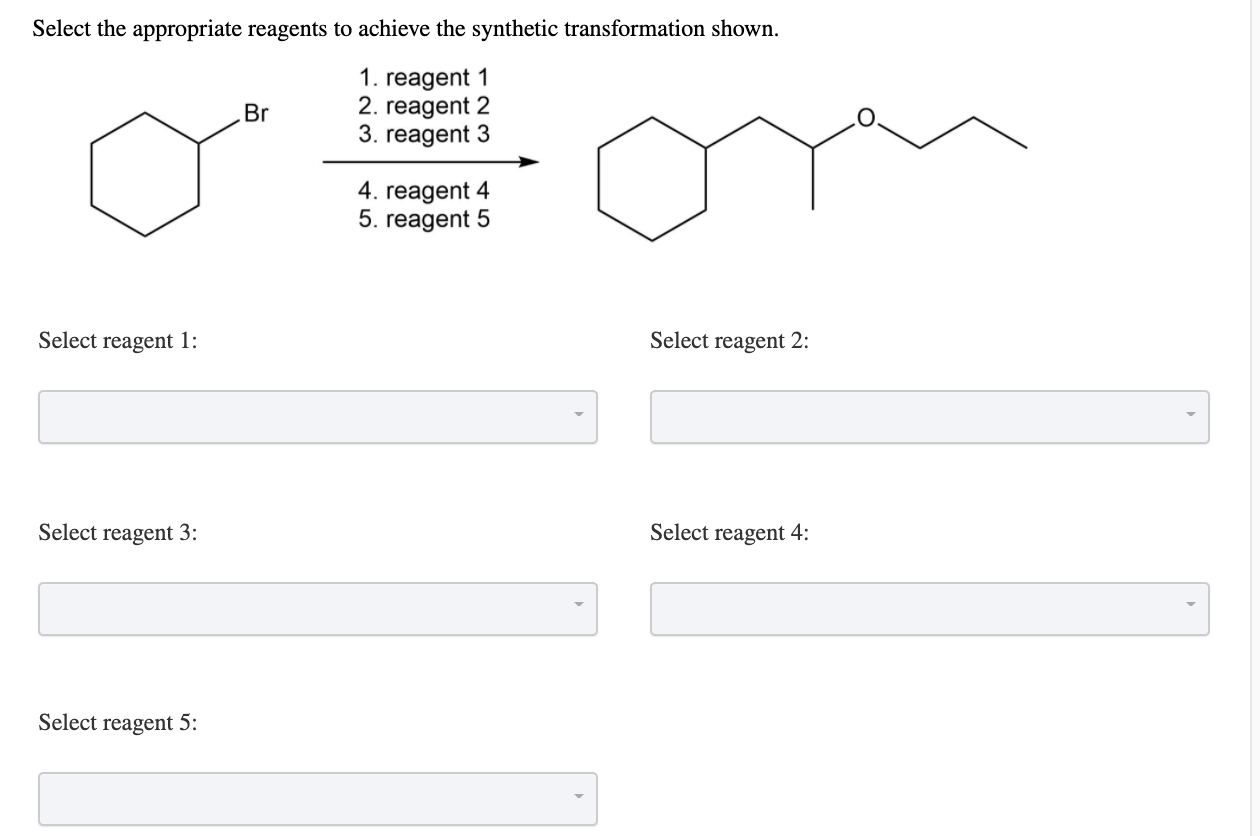
Select The Appropriate Reagents To Achieve The Synthetic Transformation Shown: Your Ultimate Guide
Chemistry can feel like a labyrinth of reactions, reagents, and transformations. But don’t worry, we’ve all been there! If you’ve ever wondered how to select the appropriate reagents to achieve the synthetic transformation shown, you’re in the right place. Today, we’re diving deep into the world of organic synthesis, breaking down the complexities into bite-sized chunks that even a rookie chemist can understand.
Imagine this: you’re staring at a reaction scheme, scratching your head, thinking, “What reagents do I need to turn this starting material into the product?” Well, my friend, that’s exactly what we’re here to solve. This guide isn’t just for the lab geeks or the PhD candidates—it’s for anyone who wants to master the art of selecting reagents like a pro.
So, buckle up because we’re about to take you on a journey through the fascinating world of synthetic chemistry. By the end of this article, you’ll be equipped with the tools, tips, and tricks to confidently select reagents that will make your synthetic transformations shine. Let’s get started, shall we?
Read also:Robert Redfords Family Life A Closer Look At His Kids And Legacy
Here’s a quick roadmap to help you navigate:
- What is Synthetic Transformation?
- Understanding Reagents and Their Role
- Common Types of Reactions in Organic Chemistry
- Choosing the Right Reagent for Your Reaction
- Factors Affecting Reagent Selection
- Practical Examples of Reagent Selection
- Troubleshooting Common Issues in Reagent Selection
- Tips for Beginners in Synthetic Chemistry
- Tools and Resources for Reagent Selection
- Conclusion: Mastering Reagent Selection
What is Synthetic Transformation?
Alright, let’s start with the basics. Synthetic transformation refers to the process of converting one molecule into another through a series of chemical reactions. It’s like turning a raw potato into fries—same ingredient, different form. In organic chemistry, this involves using specific reagents to manipulate the structure of a molecule, adding or removing functional groups, and altering its properties.
Think of it as a puzzle. Each piece represents a step in the reaction pathway, and the reagents are your tools to fit those pieces together. The goal is to achieve the desired product efficiently, selectively, and with minimal side reactions. Sounds easy, right? Well, not exactly. But with the right knowledge, it becomes a lot less intimidating.
Understanding Reagents and Their Role
So, what exactly are reagents? Simply put, they’re substances used in chemical reactions to bring about a specific transformation. They can be catalysts, nucleophiles, electrophiles, oxidizing agents, reducing agents—you name it. Each reagent has a unique role, and selecting the right one depends on the type of reaction you’re performing.
Types of Reagents
Here’s a quick rundown of the most common types of reagents:
- Nucleophiles: These are electron-rich species that attack positively charged or electron-deficient areas. Examples include hydroxide (OH⁻) and ammonia (NH₃).
- Electrophiles: These are electron-poor species that seek out electron-rich areas. Examples include bromine (Br₂) and acyl chlorides.
- Oxidizing Agents: These reagents increase the oxidation state of a molecule. Examples include potassium permanganate (KMnO₄) and chromium trioxide (CrO₃).
- Reducing Agents: These reagents decrease the oxidation state of a molecule. Examples include lithium aluminum hydride (LiAlH₄) and sodium borohydride (NaBH₄).
Now that we’ve got the basics down, let’s move on to the fun part—applying this knowledge to real reactions!
Read also:Donny Osmonds Utah Life A Heartwarming Escape From The Spotlight
Common Types of Reactions in Organic Chemistry
Organic chemistry is all about reactions, and each reaction type requires a specific set of reagents. Here’s a look at some of the most common reactions you’ll encounter:
Substitution Reactions
In substitution reactions, one group in a molecule is replaced by another. For example, in an SN2 reaction, a nucleophile attacks a substrate, displacing the leaving group. Common reagents for substitution reactions include alkyl halides, alcohols, and amines.
Addition Reactions
Addition reactions involve adding a molecule across a double or triple bond. Think of it as building something new onto the existing structure. Reagents like hydrogen bromide (HBr) and water (H₂O) are often used in these reactions.
Elimination Reactions
Elimination reactions remove atoms or groups from a molecule, usually forming a double bond. Reagents like strong bases, such as sodium hydroxide (NaOH), are commonly used to promote elimination.
Choosing the Right Reagent for Your Reaction
Now comes the tricky part—selecting the appropriate reagents. Here’s a step-by-step guide to help you make the right choice:
Identify the Reaction Type: First, determine what kind of reaction you’re dealing with. Is it substitution, addition, elimination, or something else? This will narrow down your options significantly.
Analyze the Starting Material: Look at the functional groups present in your starting material. Are there any reactive sites that need to be protected? This will influence your choice of reagents.
Consider the Desired Product: Think about the structure of your target molecule. What changes need to occur to achieve the desired transformation?
Select Compatible Reagents: Based on the above factors, choose reagents that will facilitate the desired reaction without causing unwanted side reactions.
Remember, it’s all about balance. You want reagents that are strong enough to do the job but not so strong that they cause unintended consequences.
Factors Affecting Reagent Selection
Several factors can influence your choice of reagents. Let’s explore some of the key considerations:
Reaction Conditions
The conditions under which a reaction occurs can greatly impact reagent selection. Temperature, pressure, solvent, and pH are all factors to consider. For instance, some reagents work best in acidic conditions, while others require a basic environment.
Selectivity
Selectivity refers to the ability of a reagent to target a specific functional group or site within a molecule. High selectivity is crucial for avoiding unwanted side reactions and ensuring the desired product is formed.
Safety
Safety should always be a top priority. Some reagents are highly reactive or toxic, so it’s important to choose ones that can be handled safely in the lab.
Practical Examples of Reagent Selection
Let’s put theory into practice with a few examples:
Example 1: Hydroboration-Oxidation
In this reaction, an alkene is converted into an alcohol using borane (BH₃) as the reagent. The borane adds across the double bond, and subsequent oxidation with hydrogen peroxide (H₂O₂) produces the alcohol. This is a great example of a selective and efficient transformation.
Example 2: Friedel-Crafts Alkylation
This reaction involves the introduction of an alkyl group onto an aromatic ring. Aluminum chloride (AlCl₃) is commonly used as a catalyst to facilitate the reaction. However, care must be taken to avoid over-alkylation, which can lead to undesired products.
Troubleshooting Common Issues in Reagent Selection
Even the best-laid plans can go awry. Here are some common issues you might encounter and how to address them:
Side Reactions
Side reactions occur when a reagent reacts with an unintended part of the molecule. To minimize this, consider using protecting groups to shield reactive sites or choosing more selective reagents.
Low Yield
If your reaction yields are low, it could be due to incomplete conversion or competing reactions. Adjusting reaction conditions or trying alternative reagents might help improve yield.
Tips for Beginners in Synthetic Chemistry
Starting out in synthetic chemistry can be overwhelming, but here are a few tips to help you get started:
Study Reaction Mechanisms: Understanding how reactions work at the molecular level will give you a deeper insight into reagent selection.
Practice Problem-Solving: Work through practice problems to hone your skills. The more you practice, the better you’ll become at selecting the right reagents.
Collaborate with Others: Don’t be afraid to ask for help or bounce ideas off your peers. Chemistry is a team sport!
Tools and Resources for Reagent Selection
There are plenty of tools and resources available to help you with reagent selection:
Online Databases
Websites like Reaxys and SciFinder offer comprehensive databases of chemical reactions and reagents. These can be invaluable for finding precedents and optimizing your reactions.
Textbooks
Classic textbooks like “Advanced Organic Chemistry” by Carey and Sundberg or “Organic Chemistry” by Clayden et al. provide in-depth coverage of reaction mechanisms and reagent selection.
Conclusion: Mastering Reagent Selection
Selecting the appropriate reagents to achieve the synthetic transformation shown is both an art and a science. By understanding the basics of reagents, reaction types, and selection factors, you can confidently tackle even the most complex transformations. Remember, practice makes perfect, so don’t be afraid to experiment and learn from your mistakes.
So, what are you waiting for? Grab your lab coat, put on your thinking cap, and dive into the world of synthetic chemistry. Who knows? You might just discover the next breakthrough reaction!
And hey, if you found this article helpful, don’t forget to share it with your fellow chemists. Together, we can demystify the world of organic synthesis one reaction at a time. Cheers!
Article Recommendations